
Medical devices defined in Vietnam are defined as all equipment, tools, materials and chemicals, and software that aim to provide disease testing and treatment and prevention, examination, diagnosis or mitigation, or replacement, modification or surgical assistance during examination and treatment.
The classification of medical devices in Vietnam is divided into four categories, Class A, B, C, D, depending on the risk, and if it is an overseas manufacturer, a local agent must be appointed first. The registration process is as follows.
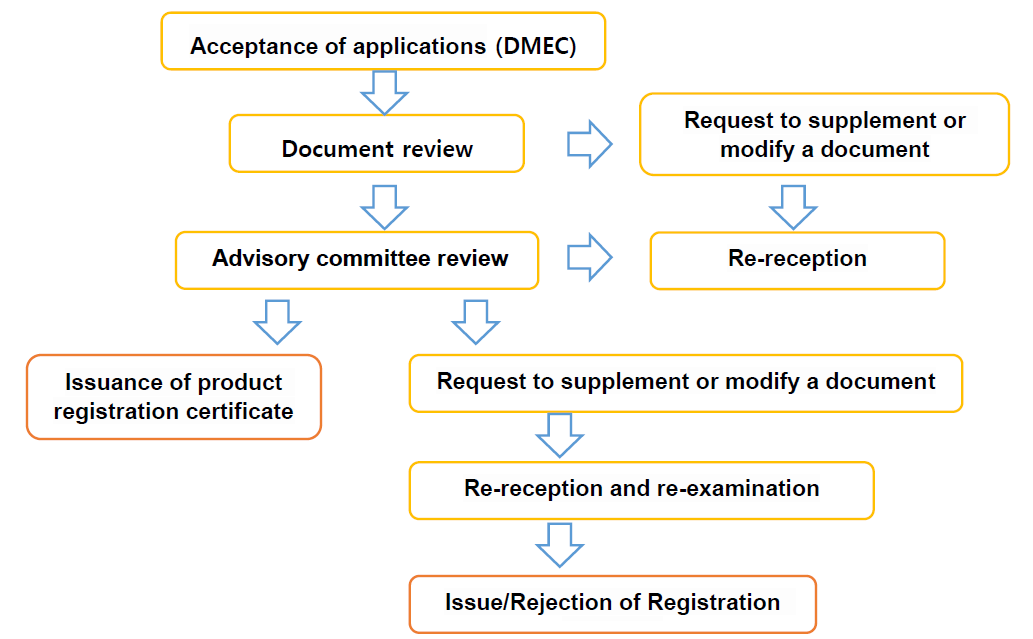
Vietnam's GMP certification is possible through ISO 13485, and if any of the U.S., Japan, Australia, Canada, EU, Korea, or China have obtained a license before exporting to Vietnam, it can proceed through the Fast Track path as an exemption from technical review.
'JNM Global > Medical Device' 카테고리의 다른 글
| UDI LABELLING MANDATORY FOR TAIWAN MEDICAL DEVICES (0) | 2023.05.19 |
|---|---|
| FDA, 510(k) Third Party Review Program (0) | 2023.05.16 |
| Services for Importing Medical Devices in Korea (0) | 2023.05.08 |
| PHILIPPINES FDA EXTENDS CMDN DEADLINE TO 2024 (0) | 2023.04.11 |
| Updated Guidance on eSTAR submissions (0) | 2023.04.11 |
